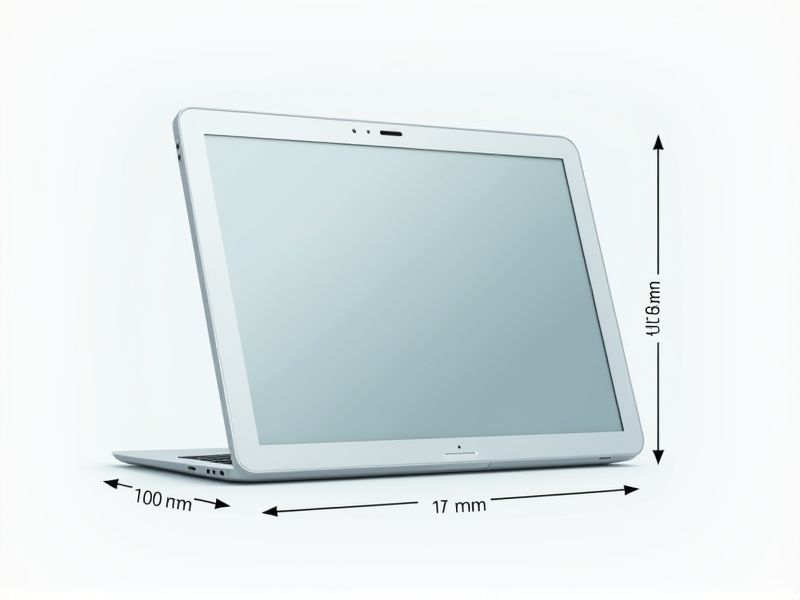
When considering the standard dimensions of a tablet, most models feature screen sizes ranging from 7 to 13 inches, measured diagonally. Popular tablets like the Apple iPad typically have a 10.2-inch display, measuring about 250.6 x 174.1 x 7.5 mm (9.8 x 6.8 x 0.3 inches). Android alternatives, such as the Samsung Galaxy Tab series, offer similar dimensions, with compact models starting at 8 inches and larger versions reaching up to 12.4 inches. Choosing the right tablet size depends on factors like portability, screen clarity, and intended use, so it's helpful to compare specifications before purchasing.
Diameter
The diameter of a standard tablet typically ranges from 6 mm to 20 mm, with most common dosages found around 10 mm. A tablet's diameter plays a crucial role in determining its ease of swallowing and patient compliance, as larger tablets can be more challenging for some individuals. For example, a typical over-the-counter pain relief tablet might measure approximately 12 mm, balancing efficacy and swallowability. When selecting or prescribing tablets, consider the diameter as a key factor to enhance user experience and medication adherence.
Thickness
Tablet thickness typically ranges from 6 to 10 millimeters, significantly impacting portability and user experience. A thinner tablet, around 6 to 8 mm, offers enhanced comfort for prolonged use and ease of transport. However, keep in mind that thickness may affect battery capacity and performance, as more powerful batteries often require additional space. When selecting a tablet, consider how its thickness aligns with your specific needs, whether that's for media consumption, gaming, or professional productivity.
Weight
Tablet weight significantly impacts portability and user experience, with most models ranging from 300 to 600 grams. The lightest tablets, such as the Apple iPad Mini, weigh around 300 grams, making them ideal for prolonged use and travel. In contrast, heavier tablets, like the Microsoft Surface Pro, can reach up to 800 grams but often offer enhanced performance features. Considering your needs, a lightweight tablet can enhance mobility without sacrificing functionality, allowing for versatile usage scenarios.
Volume
Tablets with a focus on volume typically feature advanced audio technology, offering enhanced speaker systems or Dolby Atmos support. You can expect a minimum sound output of 20 watts, providing clear and immersive audio experiences for music, videos, and gaming. Many models incorporate dual or quad speakers for richer sound quality, catering to entertainment enthusiasts. Premium tablets may also support high-resolution audio formats, ensuring that every detail in your favorite tracks is rendered crisply.
Hardness
The hardness of a tablet is critical for ensuring its structural integrity and effective performance during handling, storage, and consumption. A hardness value typically ranges between 4 to 8 KG, which is essential for preventing breakage and maintaining the correct dosage form. Tablets that are too soft may crumble, while those that are excessively hard may dissolve improperly in the gastrointestinal tract, impacting drug absorption. Regular testing of tablet hardness through instruments like hardness testers ensures compliance with pharmacopeial standards and enhances overall product quality.
Surface Area
The standard for tablet formulation heavily emphasizes surface area, as it directly influences the dissolution rate and bioavailability of the active pharmaceutical ingredient (API). Tablets with larger surface areas, typically achieved through techniques like micronization, can dissolve more quickly, enhancing absorption into the bloodstream. For optimal performance, a surface area of at least 5 m2 per gram is often targeted in manufacturing. You should consider that a well-designed tablet can significantly improve your medication's therapeutic effect by ensuring rapid and consistent bioavailability.
Friability
Friability is a critical standard in tablet formulation, representing the ability of tablets to withstand mechanical stress during handling and transport. A friability test typically allows no more than 1% weight loss for acceptable tablets, ensuring that they maintain their integrity and efficacy. High friability may indicate poor binding or formulation issues, potentially leading to disintegration problems during administration. Understanding and controlling this property significantly enhances the reliability and quality of your pharmaceutical products.
Disintegration Time
The standard for tablet disintegration time specifies that a tablet must disintegrate within a time frame of 30 minutes or less to ensure effective absorption in the body. This requirement is crucial for both immediate-release and controlled-release formulations, impacting bioavailability significantly. Pharmacopoeial guidelines set forth by organizations such as the United States Pharmacopeia (USP) provide detailed procedures for testing tablet disintegration. You should consider this metric when evaluating quality control and the overall efficacy of pharmaceutical products.
Coating
Tablet coatings significantly enhance both the stability and efficacy of pharmaceuticals by protecting active ingredients from environmental factors such as moisture and light. Over 80% of modern tablets utilize various coating technologies, including sugar, film, and enteric coatings, to improve patient compliance and taste masking. Pharmaceutical companies often invest approximately 10-20% of their production budget on coating processes to ensure the optimal release profile of the medication. Understanding the specific coating requirements for your tablet formulation can lead to improved bioavailability and targeted delivery in the gastrointestinal tract.
Compression Force
The standard for tablet compression force typically ranges from 4,000 to 12,000 Newtons, depending on the formulation and tablet size. Compression force plays a crucial role in ensuring tablet integrity, affecting characteristics such as hardness, dissolution rate, and disintegration time. Optimal force levels can enhance product quality and consistency, minimizing the risk of defects during manufacturing. By carefully monitoring and adjusting compression force, you can achieve better tablet performance and meet stringent pharmaceutical regulations.
